Background. Post-autologous stem cell transplant (ASCT) maintenance therapy for patients with newly diagnosed multiple myeloma (MM) continues to evolve. Currently, single agent lenalidomide maintenance remains standard of care but outcomes for patients with high-risk cytogenetics are inferior to those with standard risk disease; optimal maintenance strategies for these patients need to be developed. Minimal residual disease (MRD) has proven to be a powerful prognostic factor for PFS and OS but is not yet used to guide treatment. Isatuximab is a cytolytic CD38-directed, immunoglobulin G1 (IgG1) chimeric monoclonal antibody FDA-approved for the treatment of relapsed or refractory MM that is overall well tolerated and efficacious when used in combination regimens. Our upcoming trial, RAMP UP (NCT05344833), investigates the safety and efficacy of isatuximab combined with lenalidomide as a maintenance regimen in patients who are MRD-positive after autologous transplant.
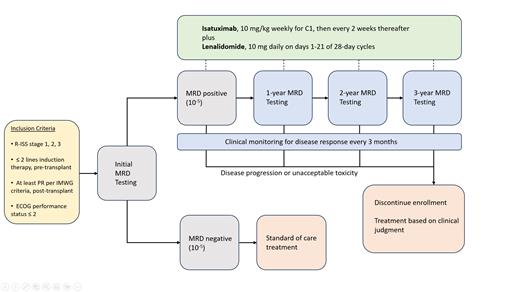
Study Design and Methods. This prospective, multicenter single-arm phase 2 (Figure 1) trial will enroll patients that are MRD-positive (10 -5) by Adaptive next generation sequencing within 90 days (+/- 30 days) post-autologous transplant. We plan to enroll: R-ISS stage 1, 2 or 3; <2 lines of induction therapy prior to transplant; > PR per IMWG criteria post-transplant; and ECOG Performance Status <2. Patients will be treated with isatuximab (10 mg/kg weekly for cycle 1 and every other week thereafter) and lenalidomide (10 mg daily on days 1-21) on 28-day cycles for a total of 3 years. Patients will be monitored for disease response every 3 months and for marrow MRD every 12 months. The primary endpoint is the 1-year rate of CR/MRD negativity (10 -5); secondary endpoints include 1-year rate of CR/MRD negativity (10 -6), IMWG-defined response rates, 3-year overall CR/MRD-negativity, PFS, OS, treatment-related adverse events, and health related quality of life (HRQoL) measured by EORTC-QLQ-C30, EQ-5D-5L, EORTC QLQ-MY20. We propose enrollment of 50 patients to allow for approximately 10% dropout rate in order to have 43 patients analyzed for the primary endpoint; for 50 patients to be treated, we estimate that 75-100 patients may have to be enrolled. Correlative objectives include correlation of isatuximab pharmacokinetics and receptor occupancy with MRD-negative response and time to progression, characterization of differences in the BM and peripheral blood T, B, and myeloid cell compartments using cellular indexing of transcriptomes and epitopes (CITE-Seq) between responders and nonresponders, and concurrent assessment of other disease biomarkers, including peripheral blood circulating multiple myeloma cells (CELLSEARCH), with MRD testing.
Discussion. Our study started June 2023. One patient has been enrolled, and two have signed informed consent for enrollment. Ultimately, our study aims to inform MRD risk-adapted approaches for maintenance therapy in myeloma.
OffLabel Disclosure:
Sweiss:Sanofi: Consultancy. Hofmeister:AbbVie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; BMS: Research Funding. Godara:Janssen: Honoraria. Rondelli:Vertex: Other: Steering Committee. Sborov:Pfizer: Membership on an entity's Board of Directors or advisory committees; Arcellx: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Abbvie: Membership on an entity's Board of Directors or advisory committees; BinayTara Foundation: Other: Support for attending meetings and/or travel; Bristol Myer Squibb: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Adaptive Biotech: Other: Payment for an educational seminar.
Isatuximab, a CD38 antibody, will be used in combination with lenalidomide as a risk-adapted maintenance strategy in patients who are MRD positive at day 90 post-autologous transplant.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal